Abstract

Introduction Effective multi-targeted drug combinations could rapidly achieve deep remissions in CLL allowing for defined duration treatment. The combination of a novel highly-specific phosphoinositide 3-kinase delta (PI3Kδ) inhibitor and casein kinase 1 epsilon (CSK1ε) inhibitor, umbralisib (UMB), and a glycoengineered chimeric monoclonal antibody (mAb) targeting a unique epitope on CD20, ublituximab (UBL), with the BCL2 inhibitor venetoclax (VEN) may decrease drug resistance, reduce risk of tumor lysis syndrome (TLS) and achieve higher levels of undetectable minimal residual disease (MRD). This triple agent therapy is being evaluated in relapsed or refractory CLL patients (U2-VEN, ClinicalTrials.gov NCT03379051). Patients received oral 800 mg UMB once-daily. For Cycle 1, UBL was given via IV on Day 1 (150 mg), Day 2 (750 mg), Day 8 (900 mg), Day 15 (900 mg), and then on Day 1 in Cycles 2 and 3 (900 mg); for 3 cycles of 28 days, followed by consolidation with UMB and VEN for cycles 4 to 24. Patient (n = 25) blood samples were collected Pre- and Post-treatment on days of UBL administration during cycles 1-3.
Our studies focused on UMB and UBL treatment effects on: 1) The decrease in the circulating CLL tumor burden and thus the subsequent risk of TLS from the addition of VEN to the regimen; and 2) CD20 antigen loss on CLL cells, a major mechanism of anti-CD20 mAb resistance.
Methods Blood was analyzed by clinical laboratory automated cell counter and then stained with antibodies against CD3, CD5, CD19, and CD20 for flow cytometry analysis. CLL cell counts were calculated from flow cytometry and clinical laboratory results. To quantitate CD20 surface molecules, peripheral blood mononuclear cells were isolated and stained for flow cytometry analysis with the addition of a Molecules of Equivalent Soluble Fluorochrome standard curve. All human specimen collection and usage was conducted with written informed consent after approval of our Institutional Research Subjects Review Board according to the ethical guidelines of the Declaration of Helsinki.
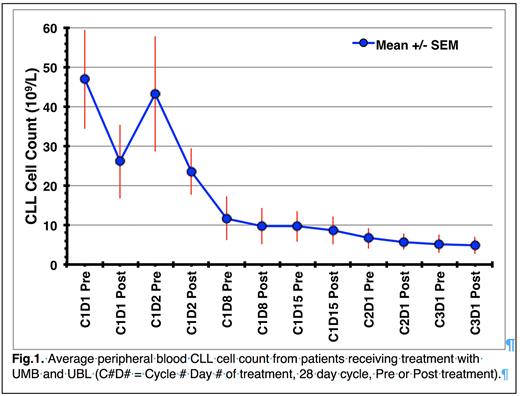
Results Average CLL cell counts (47 x 109/L) decreased rapidly after administration of the first dose of UMB and UBL (C1D1 Post, 26 x 109/L), which rebounded the next day (C1D2 Pre, 43 x 109/L) and then decreased after the second treatment (C1D2 Post, 24 x 109/L). By Day 15, the average CLL cell count had dropped to 9 x 109/L, which decreased further to 5 x 109/L by the start of cycle 3 (Fig. 1). Notably, the variation in CLL cell counts (error bars) decreases over treatment time, suggesting that most CLL patients' tumor burdens are decreasing to a similar low level independent of starting CLL cell count.
The change in average CLL cell count between Pre and Post measurements is initially very high (45% decrease C1D1) with decreasing effectiveness over treatment time (8% decrease C3D1). This change could be due to the loss of CLL cell membrane CD20 levels leading to mAb resistance. Pre-treatment (C1D1 Pre) average CLL cell membrane CD20 levels (n=18) were 29,000 CD20 molecules/cell. This decreased to 9,000 molecules/cell after the first treatment (C1D1 Post), stabilized at 9,100 molecules/cell on the next day (C1D2 Pre) and then decreased further after the second treatment (C1D2 Post) to 1600 molecules/cell. After day 8 treatment, CD20 levels remained at 1,000 molecules/cell or less until the end of this study (C3D1).
Discussion UMB and UBL rapidly decrease circulating CLL counts over three cycles of treatment, reducing most CLL patient tumor burden to similar low levels. This clinical response could facilitate addition of VEN in the 4thcycle of therapy by decreasing the risk of TLS.
The rapid loss of surface CD20 from CLL cells to 1,000 CD20 molecules/cell or less by C1D8 Post has the potential to cause acquired resistance to anti-CD20 mAb therapy. This rapid and sustained loss of CD20 levels is consistent with that reported at standard doses for other anti-CD20 mAbs. However, an effect of concomitant UMB on CD20 levels cannot be ruled out. Previous work suggests that lower anti-CD20 mAb doses can prevent these large decreases in CD20 levels and increase UBL efficacy.
In conclusion, our initial data show that treatment with a selective PI3Kδ and CSK1ε inhibitor (UMB) combined with an anti-CD20 mAb (UBL) is effective in decreasing CLL cell counts in all patients assessed in the U2-VEN clinical trial. However, treatment efficacy could be limited by large decreases in the CLL surface CD20 levels.
Disclosures
VanDerMeid:Bausch and Lomb: Current Employment. Barr:Merck, abbive, gilead, Beigene, Genentech, Astrazeneca, Janssen, TG therapeutics, Celgene, BMS, Morphosys, Adaptive: Consultancy. Zent:AstraZeneca: Research Funding; TG Therapeutics: Research Funding. Chu:Pfizer: Current equity holder in publicly-traded company.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal